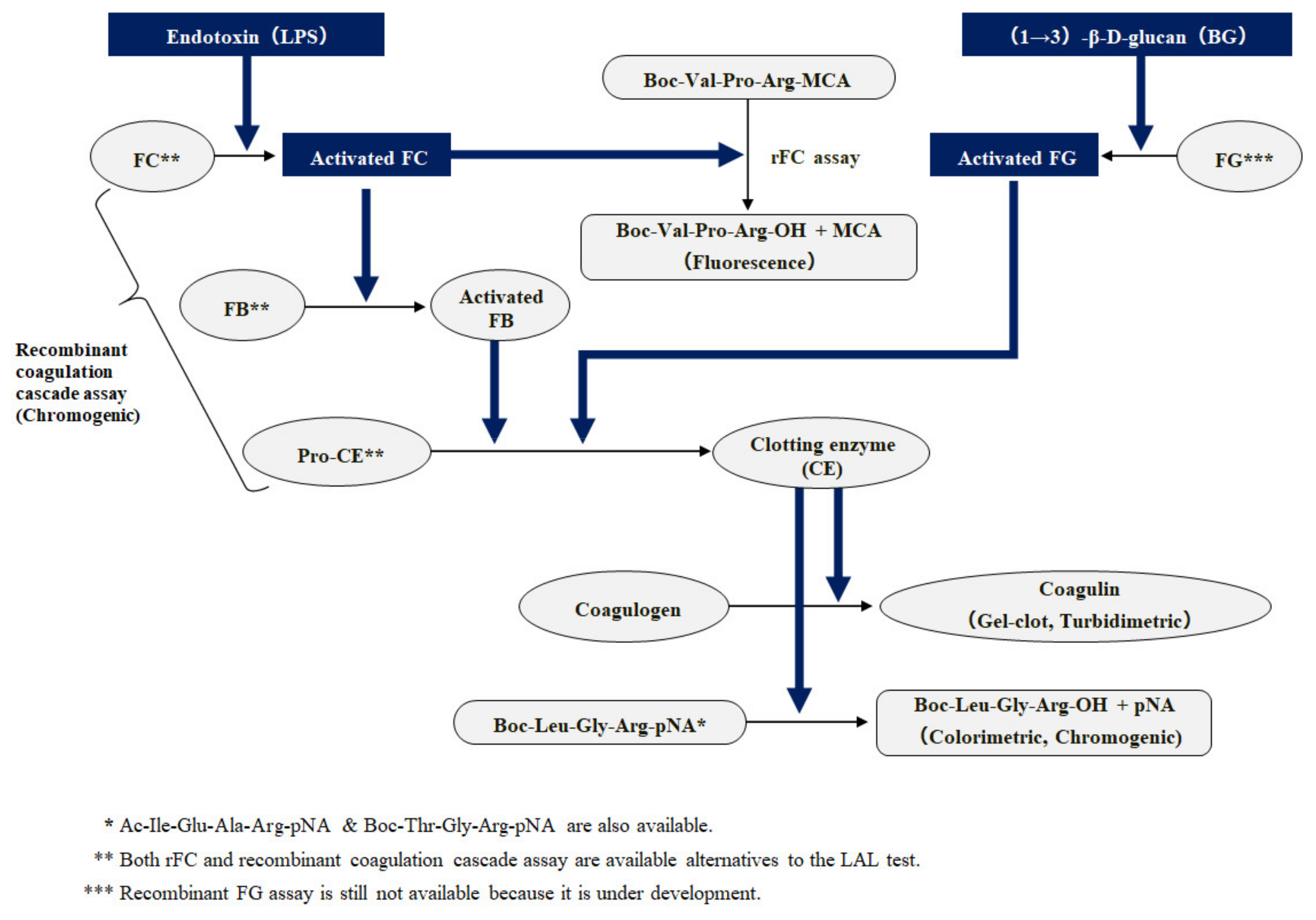
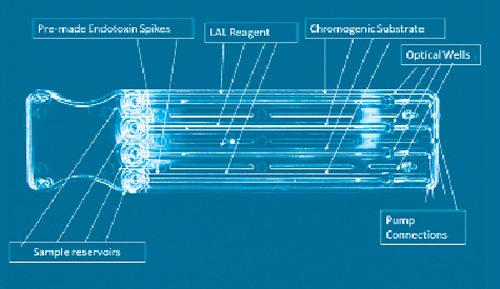
LAL test validation protocol flow-chart. The test was performed three... | Download Scientific Diagram

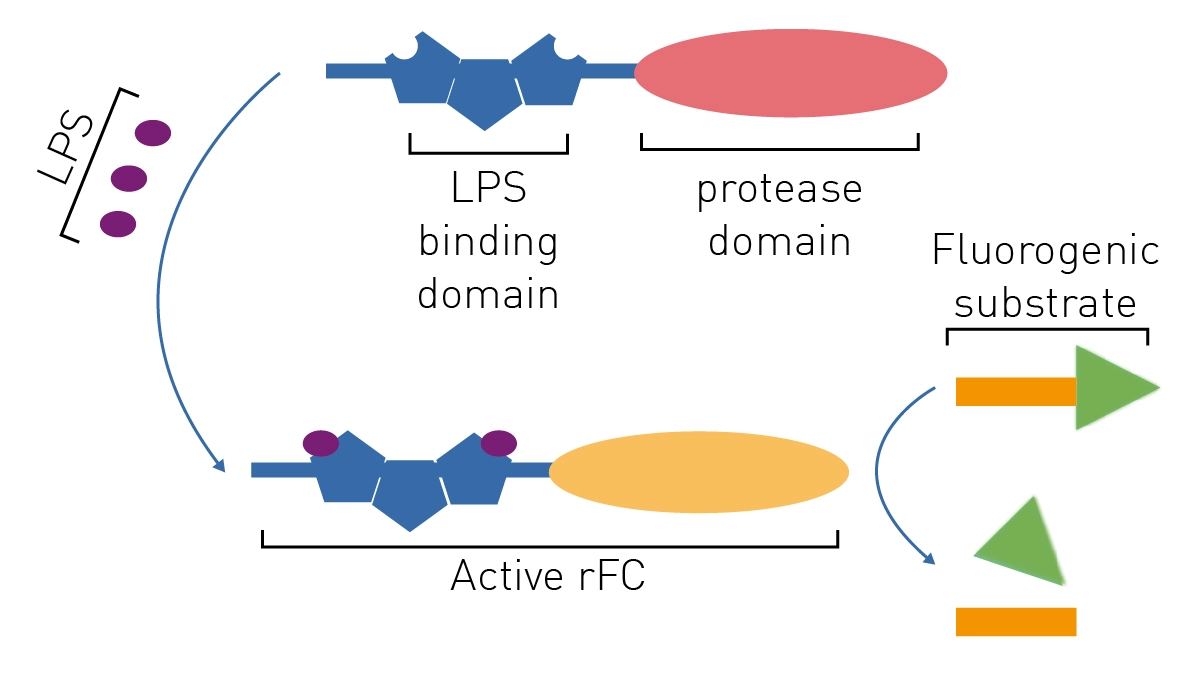
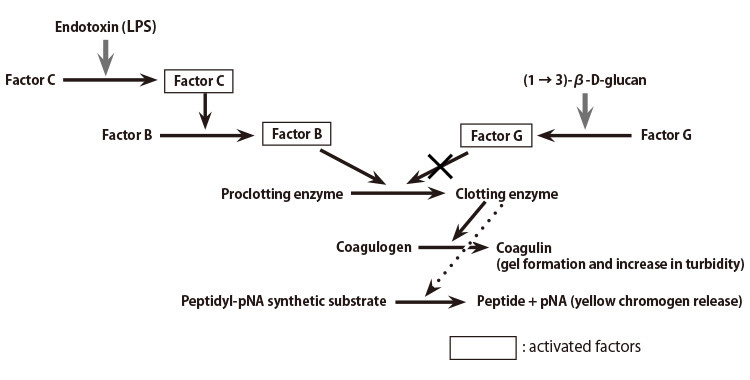
Endotoxin Testing Considerations – Reducing LAL Use with Compendial Methods | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology

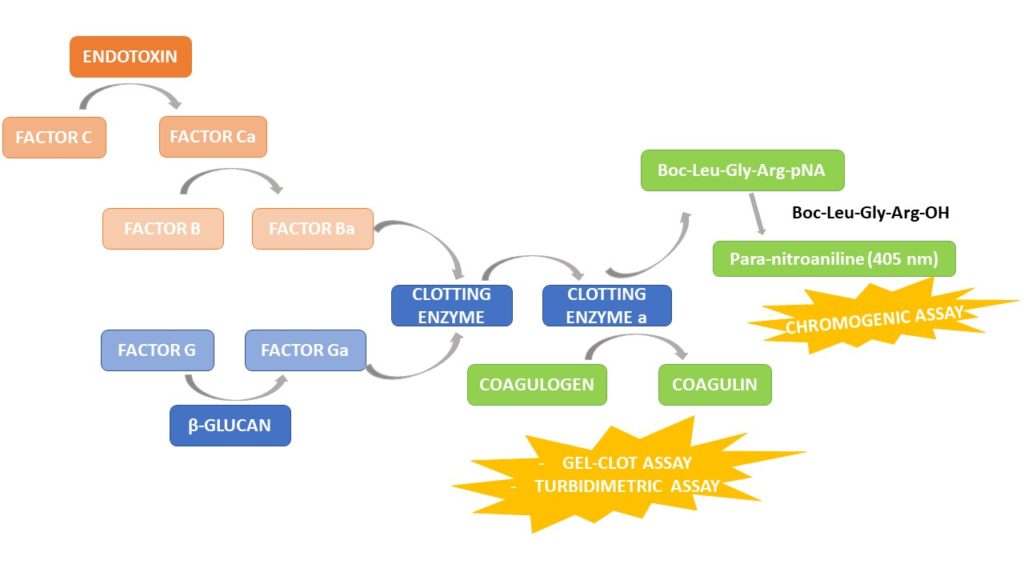
Test LAL, test dell'endotossina, reagente LAL, test del coagulo di gel, test del fattore C ricombinante, test dell'endotossina batterica, rilevamento dell'endotossina, produttori e fornitori di indicatori di endotossina - Tecnologia Bioendo

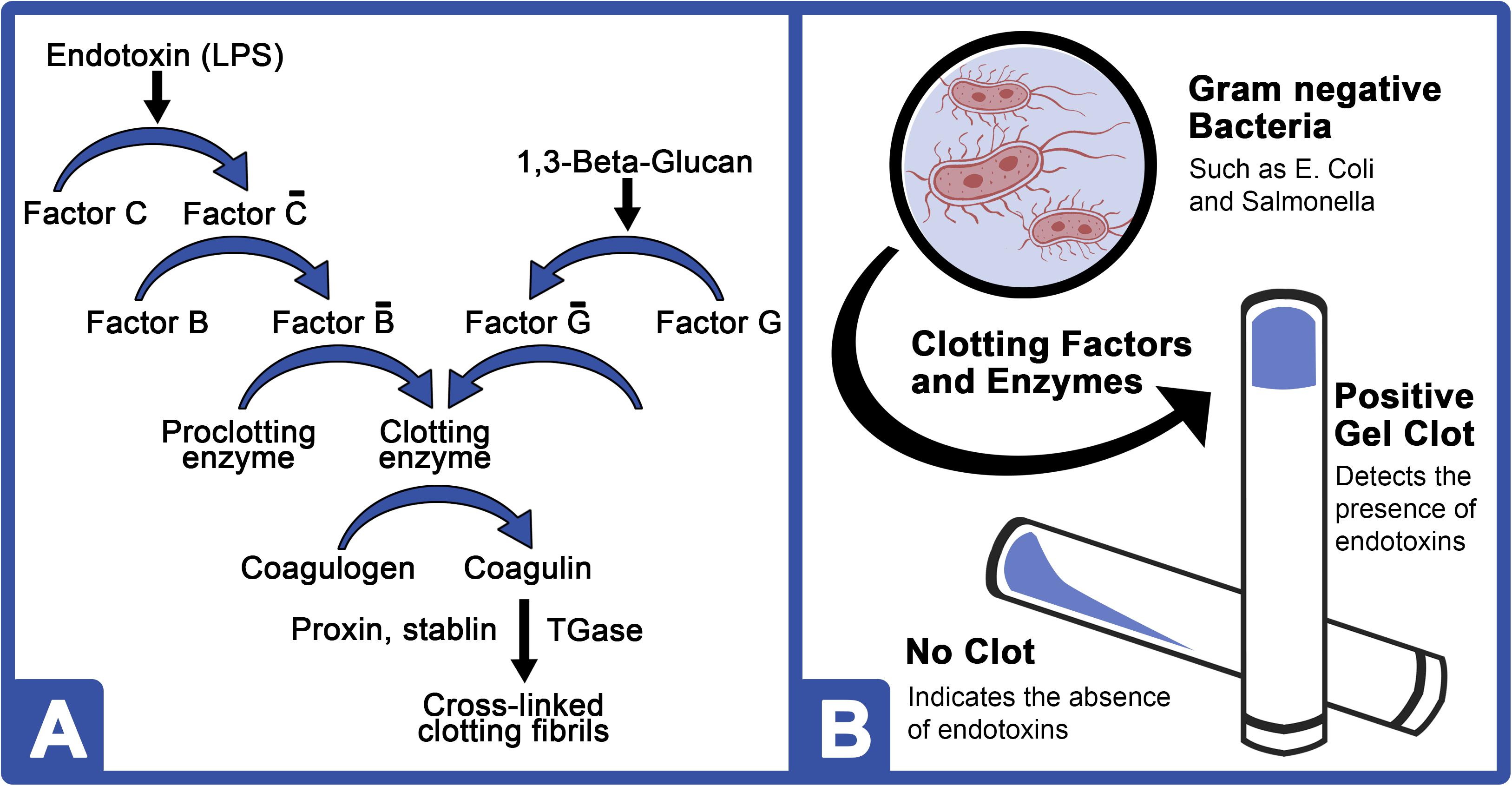
What Is Limulus Amebocyte Lysate (LAL) and Its Applicability in Endotoxin Quantification of Pharma Products | IntechOpen